Here’s what we know for certain about TH19P01: It’s a 17-amino acid peptide that binds to sortilin receptors on cancer cells. When conjugated to docetaxel as TH1902, it delivers chemotherapy directly to tumors. It works well enough to earn FDA Fast Track designation.
Here’s what we don’t know: How it was actually designed.
This gap between what we can verify and what we’re told reveals something profound about how pharmaceutical science is communicated—and why that matters for patients, investors, and researchers.
The Verifiable Facts
Let’s start with what can be independently confirmed about TH19P01:
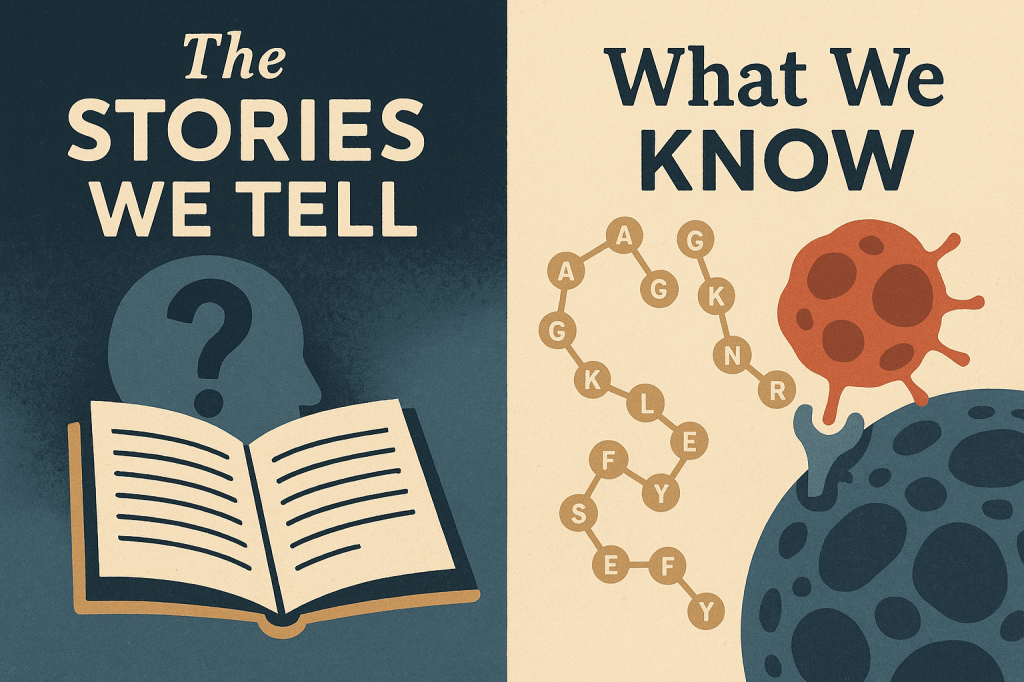
The Structure: Ac-GVRAKAGVRN(Nle)FKSESY—a 17-amino acid peptide with norleucine at position 11
The Function: Binds sortilin (SORT1), gets internalized within 4 minutes, delivers docetaxel to cancer cells
The Timeline: Developed by Katana Biopharma (2016-2019), acquired by Theratechnologies, now in clinical trials
The Clinical Reality: Some patients with sortilin-positive cancers have responded to treatment
That’s it. Everything else—every claim about how it was designed, why specific amino acids were chosen, what optimization process was used—remains unverifiable.
The Stories We Tell
Visit Theratechnologies’ website, read the scientific papers, or review analyst reports, and you’ll encounter various narratives about TH19P01’s development:
- “Engineered de novo for optimal receptor binding”
- “Proprietary sequence design”
- “Rationally designed based on sortilin structure”
- “Systematically optimized through SAR studies”
These aren’t necessarily false. But they’re not necessarily true either. They’re stories constructed after the fact to explain success.
Why We Don’t Know the Real Story
The actual design process remains hidden behind multiple veils:
Commercial Protection: Companies guard their development methods as trade secrets. Revealing exactly how TH19P01 was discovered might enable competitors.
Publication Bias: Scientific papers showcase successes, not the hundreds of failed variants that likely preceded TH19P01.
Narrative Pressure: “We got lucky with screening” doesn’t inspire investor confidence like “rationally engineered through proprietary design.”
Retrospective Coherence: Once something works, we create logical explanations for why it had to work, forgetting the role of chance and iteration.
The Probable Reality
Based on typical pharmaceutical development timelines and practices, TH19P01’s actual origin story likely involved:
- Multiple failed attempts we’ll never hear about
- Iterative optimization that was messier than any flowchart suggests
- Elements of both design and luck in proportions we’ll never know
- Post-hoc understanding of why it works
- Team debates, dead ends, and “aha” moments that don’t fit neat narratives
The three-year development timeline (2016-2019) suggests an efficient but not miraculous process—probably starting with known sortilin-binding motifs or screening data, followed by systematic optimization.
Why This Matters
You might wonder: If TH1902 helps cancer patients, who cares how it was designed?
For Patients: Understanding that successful drugs often emerge from messy, iterative processes—not just elegant design—can inform realistic expectations about drug development timelines and success rates.
For Investors: Distinguishing between verified outcomes and unverifiable origin stories enables better evaluation of platform technologies and pipeline potential.
For Scientists: Acknowledging the gap between public narratives and actual discovery processes could reduce pressure to retrofit false logic onto empirical findings.
For Drug Development: Honest discussion about the role of screening, luck, and iteration—not just rational design—might improve how we approach and fund discovery.
A More Honest Framework
Instead of pretending we know how drugs were designed, we could adopt more honest language:
❌ “TH19P01 was rationally designed to…” ✅ “TH19P01, developed through proprietary methods, successfully…”
❌ “The team engineered the peptide by…” ✅ “The peptide’s structure suggests optimization for…”
❌ “Based on structural insights, they created…” ✅ “The final sequence achieves…”
The Bottom Line
TH19P01 works. It binds sortilin, enters cancer cells, and delivers chemotherapy. For patients, that’s what matters. The peptide’s actual design story—whether brilliant rational engineering, lucky screening hit, or something in between—remains locked in Katana Biopharma’s lab notebooks and the memories of its creators.
Perhaps the most important lesson from TH19P01 isn’t about peptide design or cancer targeting. It’s about scientific honesty: recognizing the difference between what we can verify and the stories we tell, and being comfortable saying “we don’t know” when we don’t.
In an era of breakthrough hype and investment speculation, that kind of honesty might be the most radical innovation of all.

