A novel lasso peptide antibiotic with a unique twisted structure is demonstrating encouraging preliminary results against drug-resistant bacteria, though significant development challenges remain
The Discovery in a Backyard
Sometimes promising scientific discoveries come from unexpected places. When Dr. Gerry Wright’s lab technician collected soil from his Hamilton, Ontario backyard, it yielded bacteria producing something potentially significant: lariocidin, the first ribosome-targeting member of the lasso peptide antibiotic family.
What makes lariocidin noteworthy isn’t just its preliminary antimicrobial activity—it’s the remarkably unique way this molecule is built. Like a molecular knot, lariocidin features a “lasso” structure that may provide properties different from conventional antibiotics, though clinical relevance remains to be determined.
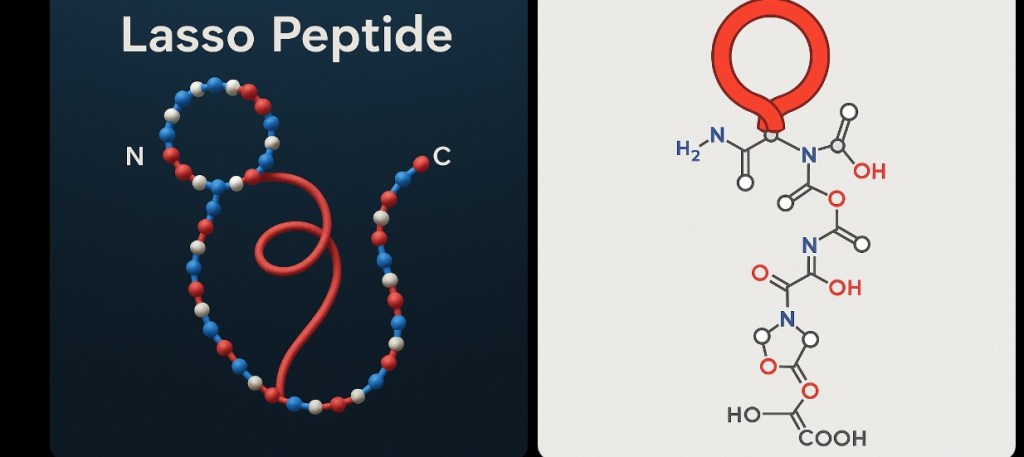
Understanding the Lasso: Nature’s Molecular Knot
Imagine threading a rope through a loop and pulling it tight—that’s essentially what nature does when creating lasso peptides. The structure consists of:
- A ring formed by the first 7-8 amino acids
- A tail that threads through this ring and extends beyond it
- An isopeptide bond that locks the ring in place
- A slipknot topology that creates enhanced stability
This isn’t just structurally interesting—it appears functionally relevant. The threaded structure makes lasso peptides notably stable, resistant to heat, and difficult for bacterial enzymes to degrade in laboratory conditions. Whether this translates to clinical advantages remains unclear.
Early Resistance Studies Show Promise
Here’s where lariocidin shows encouraging preliminary results: in 30-day laboratory studies, bacteria showed minimal resistance development compared to other antibiotics tested under similar conditions. While promising, this represents a very limited timeframe in the context of bacterial evolution.
Important caveats:
- Resistance studies were conducted for only 30 days
- Laboratory conditions may not reflect clinical reality
- Historical precedent shows bacteria eventually develop resistance to all antibiotic classes given sufficient time and evolutionary pressure
- Longer-term studies (6+ months) and clinical data are needed for meaningful conclusions
The potential resistance advantages may stem from:
Structural Constraints
The lasso structure appears sensitive to modification—mutations that might provide resistance could disrupt the essential threading mechanism, though this hypothesis requires validation.
Novel Target Strategy
Lariocidin targets a previously unexploited site on bacterial ribosomes. Unlike other antibiotics that attack well-studied ribosomal locations where resistance mechanisms are established, lariocidin found what appears to be virgin territory.
Specifically, it binds to a unique site in the small ribosomal subunit where it:
- Blocks protein synthesis by preventing ribosomal movement
- Causes translation errors by inducing misreading of genetic instructions
- Employs dual mechanisms simultaneously
The Antibiotic Resistance Crisis Context
To understand why lariocidin’s early results matter, consider our current situation. We face growing challenges where common infections are becoming harder to treat due to antibiotic resistance. The WHO lists antibiotic-resistant bacteria among significant global health concerns.
Most “new” antibiotics are modifications of existing drugs—incremental improvements rather than fundamental innovations. Bacteria have had decades to adapt to these approaches.
Lariocidin represents a genuinely different structural approach, though whether this translates to clinical success remains highly uncertain.
Preliminary Laboratory Performance
Early testing reveals lariocidin’s potential capabilities, though with important limitations:
Laboratory Activity Against:
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Vancomycin-resistant Enterococcus (VRE)
- Carbapenem-resistant Acinetobacter baumannii
- Other drug-resistant pathogens tested
Preliminary Safety Observations:
- No cytotoxicity observed in initial cell culture studies at tested concentrations
- Appears selective for bacterial vs. human ribosomes in laboratory assays
- Critical limitation: No human studies have been conducted; in vitro safety doesn’t predict clinical safety
Animal Model Results:
- 100% survival in mouse models of A. baumannii infection
- Important context: Sample size and study details not disclosed; mouse models frequently fail to predict human outcomes
- Single infection model tested; broader efficacy unknown
The Lasso Structure: Potential Advantages
The lasso peptide structure may provide several benefits, though clinical relevance is unproven:
1. Enhanced Stability
The knotted structure shows resistance to:
- Heat degradation in laboratory conditions
- Protease digestion in test systems
- pH variations in controlled studies
2. Constrained Conformation
The structure appears to maintain:
- Consistent three-dimensional shape
- Predictable binding interactions in laboratory assays
- Reproducible activity in test conditions
3. Potential Resistance Barriers
The complex topology might mean:
- Resistance mutations could disrupt essential structure
- Multiple simultaneous changes might be required
- Caution: This remains theoretical; bacteria are remarkably adaptable
4. Production Feasibility
Despite structural complexity:
- Can be produced through bacterial fermentation
- Gram-per-liter yields reported in laboratory conditions
- Scale-up challenges remain unexplored
Beyond Lariocidin: Related Discoveries
The research has revealed additional variants:
Lariocidin B: The Double Lasso
A more complex variant with two threading points shows enhanced stability in laboratory conditions. Whether this improved stability translates to therapeutic advantages is unknown.
Broader Lasso Peptide Family
Nature produces hundreds of lasso peptides, most unexplored for antibiotic potential. Each represents a possible research target, though most natural products fail to become drugs.
The Long Road Ahead
Lariocidin faces the standard drug development journey with its inherent uncertainties:
Current Status:
- Preclinical development stage
- Initial toxicology studies planned
- Formulation challenges being addressed
Potential Timeline (highly uncertain):
- IND-enabling studies: potentially 2025-2026, if funding secured
- First-in-human trials: possibly 2026-2027, if preclinical studies succeed
- Clinical development: 2027-2032 optimistically, if trials are positive
Development Challenges:
- Limited funding for antibiotic research
- Uncertain regulatory pathways for new antibiotic classes
- Manufacturing complexity for constrained peptide structures
- Historical reality: 90% of promising preclinical drugs fail during development
A Cautious Perspective on Progress
Lariocidin represents encouraging early-stage research that warrants continued investigation. The lasso peptide structure demonstrates that natural products continue to provide novel molecular architectures for exploration.
However, the path from promising laboratory results to approved therapy is exceptionally challenging. Most potential antibiotics fail during development due to toxicity, lack of efficacy, manufacturing issues, or regulatory obstacles.
While the preliminary resistance data is encouraging, history shows that bacteria eventually adapt to virtually all antimicrobial approaches given sufficient evolutionary pressure and time.
What This Means for Future Research
The early success of lariocidin studies may encourage renewed interest in natural product antibiotic discovery, particularly exploration of structurally novel peptide families.
The research reminds us that evolution has been conducting molecular experimentation for millions of years, occasionally producing structures worth investigating. However, translation from natural product to therapeutic agent remains one of the most challenging endeavors in drug development.
As Dr. Wright noted: “This is a great example of how synergistic skills and research funding come together to help solve some of these big problems that we’re facing as a society.”
The collaborative research continues, with the understanding that promising early results represent just the first step in a long, uncertain journey toward potentially helping patients.
The Realistic Outlook
Lariocidin’s story illustrates both the promise and challenges of antibiotic discovery. While the early laboratory results are encouraging and the novel structure provides reason for optimism, significant scientific, regulatory, and commercial hurdles remain.
The research team’s careful, systematic approach to development—combined with realistic expectations about the challenges ahead—offers the best chance for this promising early discovery to potentially contribute to our future antimicrobial arsenal.
Whether lariocidin ultimately succeeds or joins the long list of promising compounds that didn’t reach patients, the research advances our understanding of novel antimicrobial mechanisms and provides valuable insights for future drug discovery efforts.
Research on lariocidin continues through partnerships between McMaster University and the University of Illinois Chicago. The findings were published in Nature and represent the first ribosome-targeting lasso peptide antibiotic discovered, though clinical significance remains to be determined through future studies.

